Organic synthesis
The "organic synthesis" part focuses on obtaining original heterocycles, playing the role of skeletons onto which a wide variety of groups will be grafted by means of methods developed in the laboratory. Our work focuses on the preparation of heterocyclic systems mainly nitrogen- or oxygen-containing cycles. In a large number of cases, obtaining these chemical entities requires the use of reactions catalyzed by transition metals (palladium, copper, gold, platinum). The preparation of these heterocycles is intrinsically associated with the development of methodologies allowing their functionalization to study the influence of these modifications on the biological activity of our derivatives but also on their pharmacokinetic properties and their toxicity.
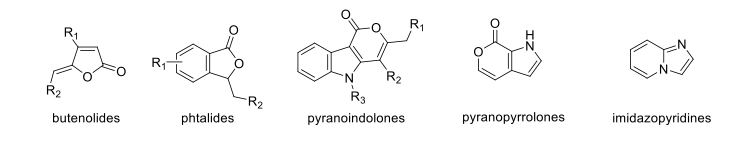
Examples of heterocycles
Examples of syntheses developed in the laboratory
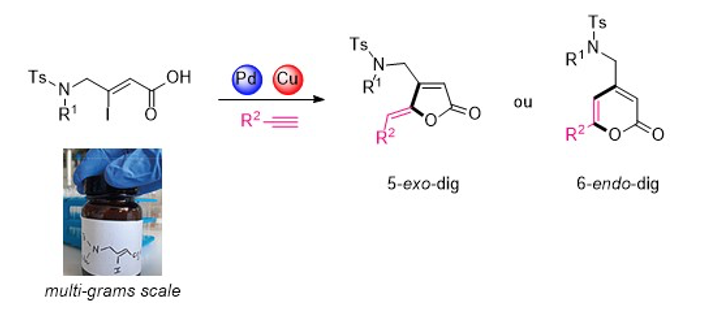
Synthesis of lactones carrying nitrogenous side chains (Eur. J. Org. Chem. 2019, 7439-7447)
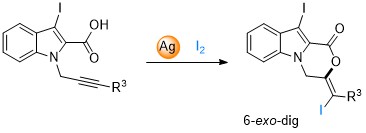
Synthesis of oxazinoindolone by regioselective iodolactonization (Eur. J. Org. Chem. 2018, 6314-6327)
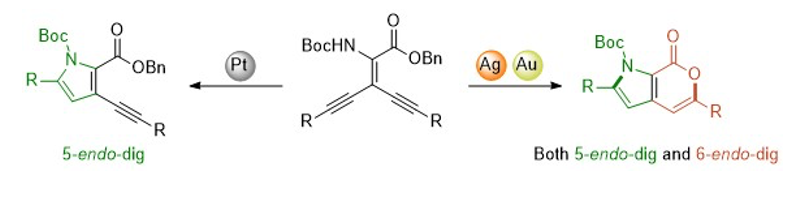
Gold catalyzed Synthesis of pyrano [3,4-b] pyrrol-7 (1H) -ones (Org. Biomol. Chem., 2017, 15, 7290-7295)
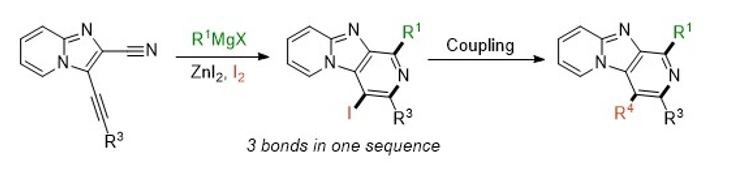
Synthesis of imidazo [1,2-a: 4,5-c '] dipyridines via a Grignard addition / cyclization / halogenation sequence (Synthesis, 2015, 47(24), 3983-3989)
Applications to the total synthesis of natural products
Our research also focuses on the total synthesis of natural molecules comprising these heterocyclic units. This approach makes it possible to confirm the chemical structures of the molecules described in the literature, but also the preparation of new analogues of these natural products with a view to improving their possible biological properties.
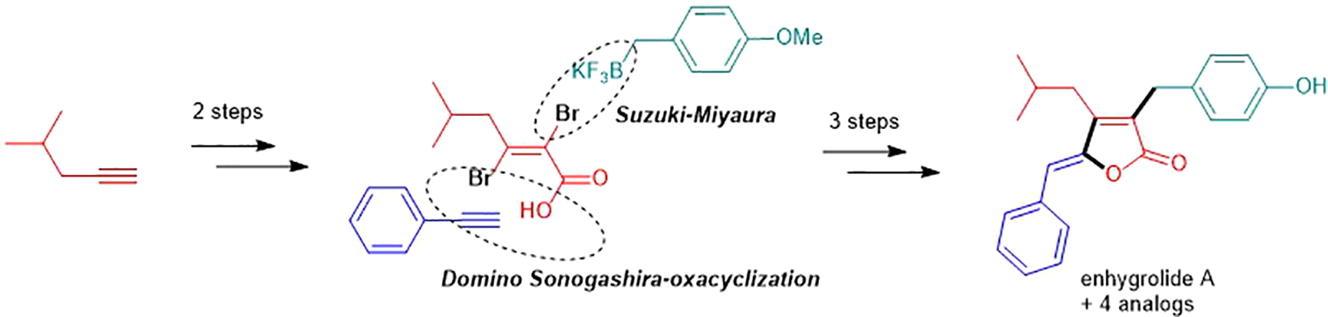
Total synthesis of enhygrolide A and analogues (Tetrahedron Lett. 2020, 61 (16), 151786)
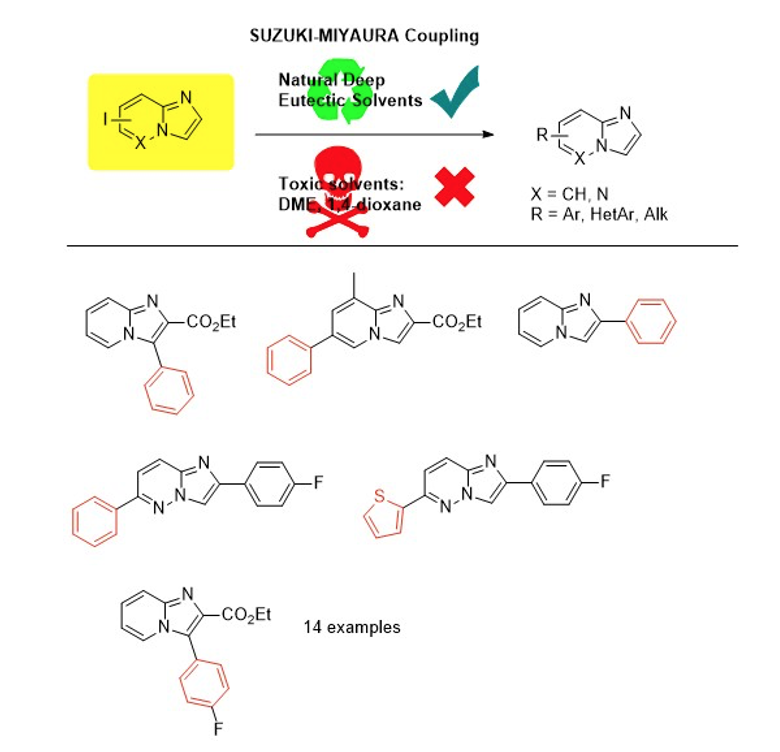
Green chemistry: use of deep eutectic solvents (DES)
Our team is also increasingly interested in the use of Deep Eutectics Solvents (DES) in organic synthesis as an alternative to conventionally used organic solvents. The objective is to use solvents with less environmental impact.Use of DES for Suzuki-Miyaura couplings applied to heterocycles of the imidazopyridine and imidazopyridazine type (SynOpen, 2018, 02(04), 0306)