Cancerology
Molecules of the imidazo [1,2-a: 4,5-c '] dipyridine type, inspired by natural β-carbolines such as harmonine (alkaloid with anti-cancer properties) are evaluated for their anti-proliferative and anti-migration properties, on breast cancer cell lines.
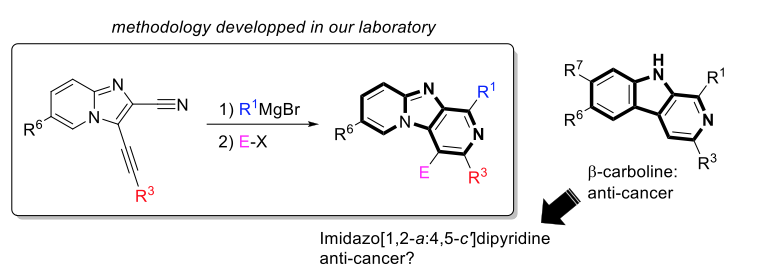
The access route to these structures, developed in the laboratory, allows the preparation of numerous analogues variously substituted at positions 1, 3, 4, 6. Several "leads" have been identified in vitro, with IC50s of up to 0.1µM on the MDA-MB-468 lines. More interestingly, some molecules in this series have the ability to inhibit the migration of MDA-MB-435 cancer cells, very aggressive and highly metastatic cells (up to 62% inhibition at 1 µM). The project is funded by the Ligue contre le Cancer, and mechanistic studies, as well as in vivo studies, are currently underway.
In addition, Lamellarins, isolated from marine molluscs, have proven to be a family of compounds of particular interest for the treatment of cancer. Our team has recently developed an original and efficient strategy for the preparation of the pyrano [3,4-b] pyrrol-7 (1H) -one unit, thus allowing the obtaining of simplified analogues of Lamellarins.
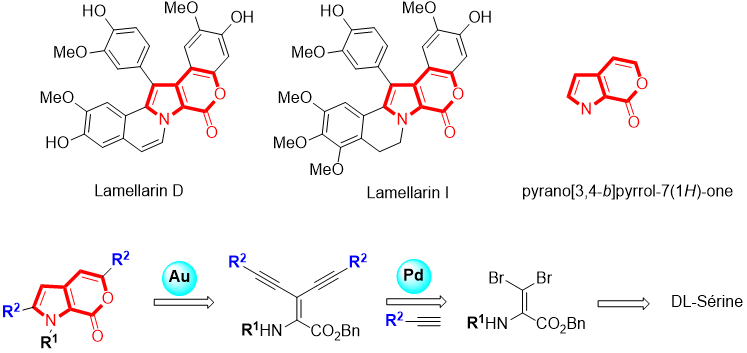
The evaluation of the pro-apoptotic activity of the first compounds with respect to the mitochondrial pathway was carried out in collaboration with the team of Dr. Jérôme Kluza from the UMR-S 1172 laboratory (Inserm, University of Lille) and has shown that certain molecules synthesized exhibit cytotoxicity equivalent to lamellarin D. We are currently studying the pharmacomodulations to be implemented on the pyrano [3,4-b] pyrrol-7 (1H) -one backbone in order to improve the activity pro-apoptosis and selectivity towards tumor cells.
